- Lewis Dot Structure For Ionic Compounds Calculator Compound
- Lewis Structure For Ionic Bond
- Lewis Dot Structure For Al
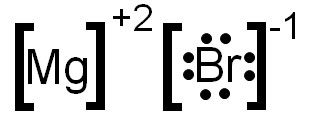
Lewis Dot Structure For Ionic Compounds Calculator Compound
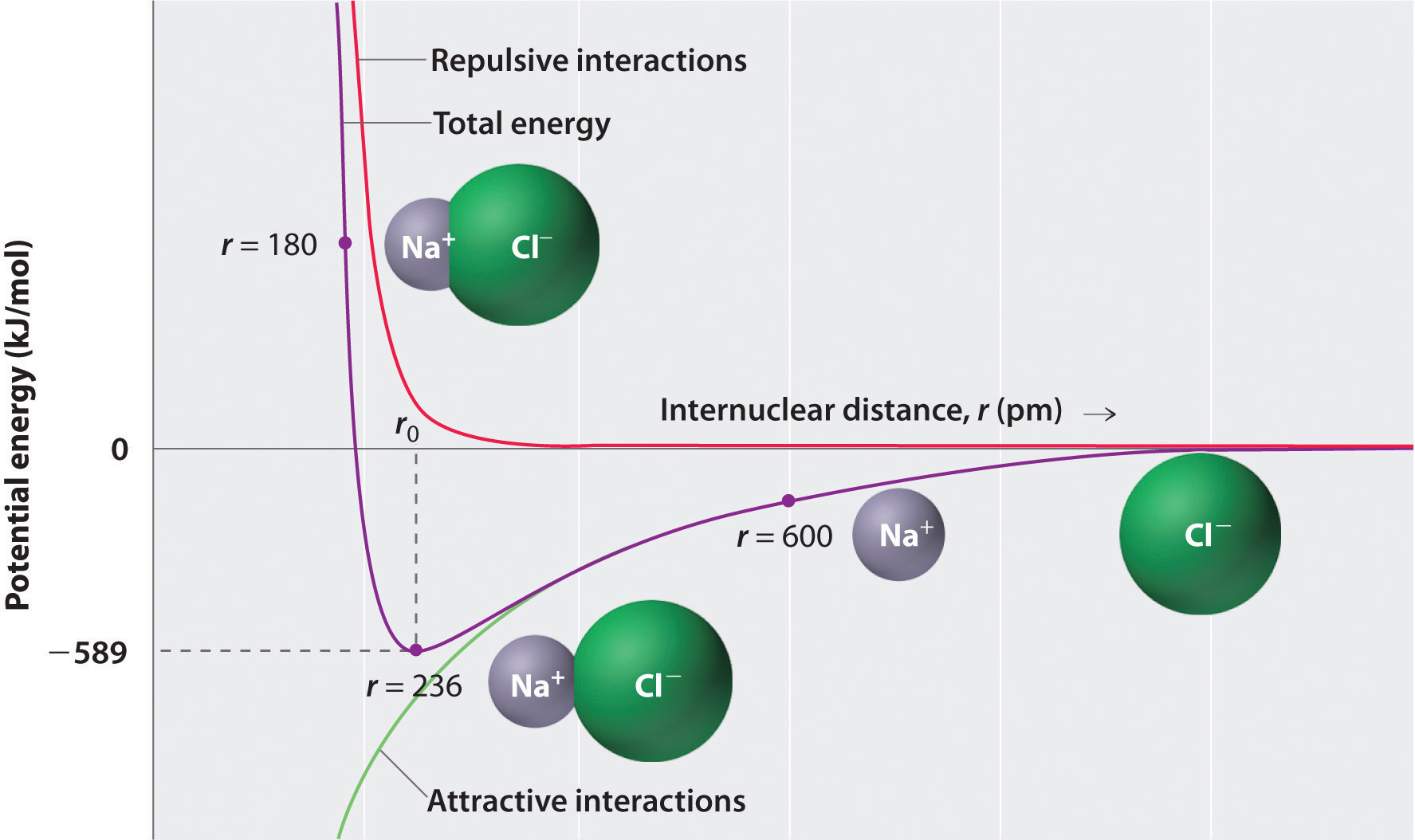
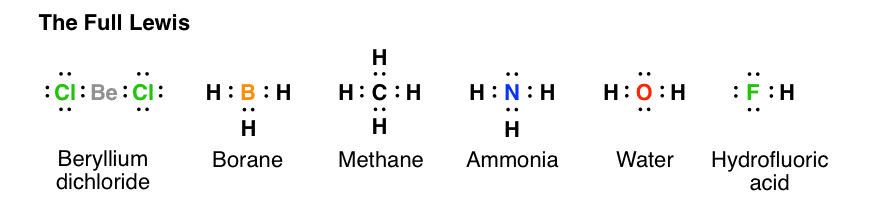
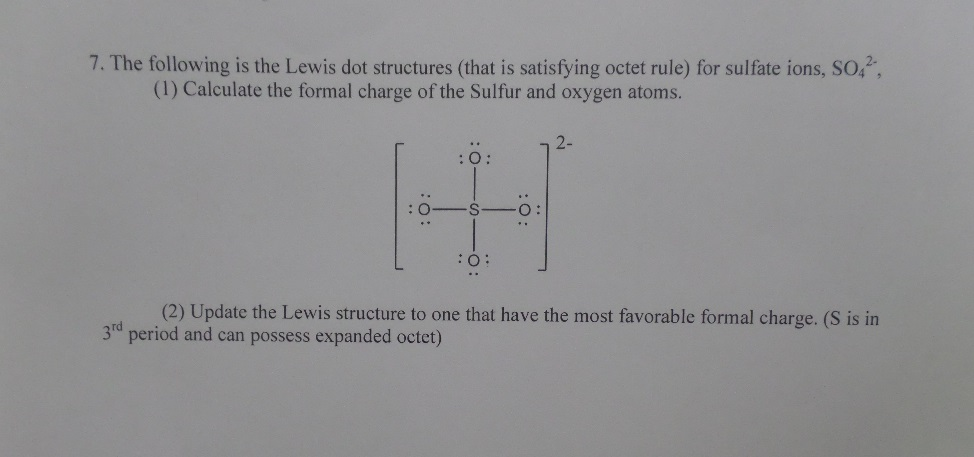 * In the formation of Dinitrogen molecule, each nitrogen atom contributes 3 Figure 7.12 shows the Lewis structures for two hypervalent molecules, PCl5 and SF6. Question to ponder: What is the overall polarity of the carbon dioxide 12.4: Covalent Bonds and Lewis Structures, [ Complete the octets around the surrounding atoms (except for H). * The ...In this interactive and animated object, students distribute the valence electrons in simple covalent molecules with one central atom. Six rules are followed to show the bonding and nonbonding electrons in Lewis dot structures. The process is well illustrated with eight worked examples and two interactive practice problems.
* In the formation of Dinitrogen molecule, each nitrogen atom contributes 3 Figure 7.12 shows the Lewis structures for two hypervalent molecules, PCl5 and SF6. Question to ponder: What is the overall polarity of the carbon dioxide 12.4: Covalent Bonds and Lewis Structures, [ Complete the octets around the surrounding atoms (except for H). * The ...In this interactive and animated object, students distribute the valence electrons in simple covalent molecules with one central atom. Six rules are followed to show the bonding and nonbonding electrons in Lewis dot structures. The process is well illustrated with eight worked examples and two interactive practice problems. 
Lewis Structure For Ionic Bond
- Ionic compounds are made when one element loses electron(s) to become a positive ion, and the other element gains electron(s) to become negative ions. You would show the electrons in the outer level in the diagram eg Na+:Cl:- with another 2 dots above the Cl and two more below the Cl making 8 electrons in total on te Cl- ion.
- The Lewis dot structure would be Cr with one dot over it. Lewis structure, electron dot diagram, electron dot structure Asked in Chemical Bonding How do you draw a Lewis dot structure for an ionic compound? How do you draw a lewis dot structure for an ionic compound? It looks like a lewis dot structure for C2H2F2.One of the early questions.
- Electron dot structures or Lewis dot formula can be drawn if the molecular formula of the compound is known. It defines the nature of bond and position of atoms of the molecule which are connected in the molecule.
- Given descriptions, diagrams, scenarios, or chemical symbols, students will model ionic bonds using electron dot formulas.
Lewis Dot Structure For Al
Nov 24, 2018 Use information from step 4 and 5 to draw the CH 4 lewis structure. Easy Way – Treat them like Puzzle Pieces Lewis structure of CH 4. Alternatively a dot method can be used to draw the CH 4 Lewis structure. Calculate the total valence electrons in the molecule. Total=8; This unit is part of the Chemistry library.